2na S 2h2o L 2naoh Aq H2 G
Start your trial now. Write down all the ions present.
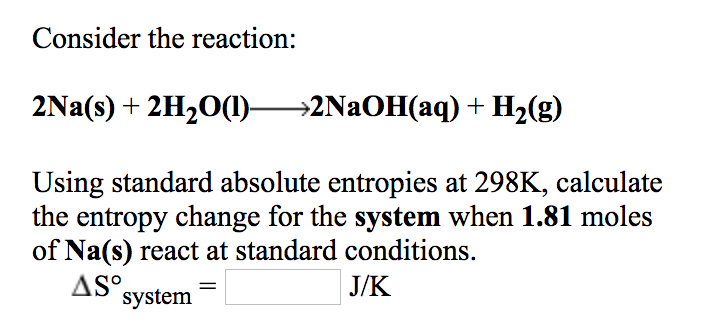
Solved Consider The Reaction 2na S 2h2o 1 2naoh Aq Chegg Com
Mass of Aluminum Foil is 0450g Mass of Alum after drying is 6852g What is the theoretical yield.
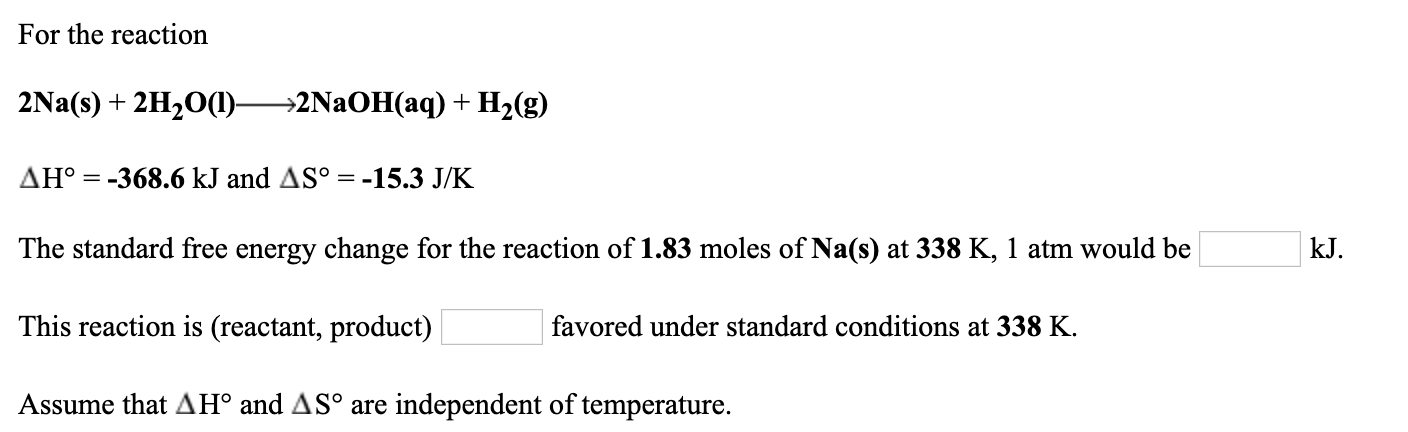
. What type of reaction is illustrated. Ecuación química balanceada. Calculate the work done in joules by the reaction 2Nas 2H2Ol 2NaOHaq H2g.
Natrium hidroksida juga dikenal sebagai lindi lye dan soda kaustik atau soda api adalah suatu senyawa anorganik dengan rumus kimia NaOH. N2 3H2 2NH3. Write down the full balanced equation.
What are the spectator ions in this reaction. Skip to main content. This reaction is reactant product favored under standard conditions at 266 K.
H2 2NaOH Blank 1 - 2 Blank 2 -2 Blank 3 -1 Blank 4 -2 question_answer Q. Na H2O NaOH H2. Natrium hidroksida merupakan basa dan alkali yang sangat kaustik mampu menguraikan protein pada.
Any reactant or product that has a state symbol s l or g or is a covalent molecule in solution such as chlorine Cl2aq does not split into ions. Enter the email address you signed up with and well email you a reset link. В началото на следващия исторически период наричан каменно-медна епоха са се използвали мед и камък за направата на.
The French word titre also comes from this origin meaning rank is a common laboratory method of quantitativechemical analysis that can be used to determine the concentration of a known reactant analyte. Puedes utilizar los paréntesis o los corchetes. CH4l 2O2g - CO2g 2H2Og can be classified as A oxidation-reduction reactions You have exposed electrodes of a light bulb in a solution of H2SO4 such that the light bulb is on.
Mgs 2Haq 2Claq Mg2aq 2Claq H2g Step 3. CH4 2O2 CO2 2H2O 1 Mol. 2Na2H2O2NaOHH2 The equation is balanced.
Медта е първият метал който е бил известен след палеолитния и неолитния период. Molecular equation complete ionic equation net ionic equation. Na H2O Al NaAlO2 H2.
2NaCl 2H2O 电解 2NaOH H2 Cl2 2NaCl熔融 电解 2Na Cl2 以上就是科学高分网小编总结的化学方程式的书写规范希望同学们能够牢记以上这些书写规范再写化学方程式的过程中避免不必要的失分. Sodium hydroxide は化学式 NaOH で表される無機化合物でナトリウムの水酸化物であり常温常圧ではナトリウムイオンと水酸化物イオンからなるイオン結晶である 苛性ソーダかせいソーダ英. For the reaction 2 HO1 2 H2g Og AG 4847 kJ and AH - 5716 kJ at 266 K and 1 atm.
NaOHaq 2 mol-47011 kJmol-94022 kJ. 2 2 2 1. Since there is an equal number of each element in the reactants and products of 2Na 2H2O 2NaOH H2 the equation is balanced.
Na H2O NaOH H2 - Balanceador de Ecuaciones Químicas. The word titration comes from the Latin titalus meaning inscription or title. Enter the email address you signed up with and well email you a reset link.
Solution for How much work w must be done on a system to decrease its volume from 170 L to 80 L by exerting a constant pressure of 60 atm. First week only 499. 2Na 2H2O --.
2由于 HNO3 具有强氧化性 H2S 具有还原性此反应为 HNO3 H2S氧化成 S自身 被还原为 NO其中 H2 时生成32 S当转移03 mol 电子时得到 质量为48 均具有还原性当通入少量Cl2 时只有 Fe 被氧化说明还原性Fe 当通入足量Cl 完全被氧化离子方程式. S2 HaqMg2aqH2g The equation that contains spectator ions is called the. Mgs 2HClaq MgCl2aq H2g Step 2.
2 Na 2 H 2 O 2 NaOH H 2. Aqueous potassium carbonate reacts with aqueous silver nitrate to precipitate silver carbonate. What volume of H2 gas measured at 21 degrees Celsius and 689 torr can be obtained by reacting 629 g of zinc metal with 131 mL of 0245 M HCl.
No se requieren los estados compuestos como s aq o g. What is the net ionic equation of this reaction. The entropy change for the reaction of 156 moles of H0 at this temperature would be Consider the reaction COg Clg-COC1g Using the standard thermodynamic data in the.
Na H2O Al BaOH2 BaNaAlO22 H2. Senyawa ini merupakan senyawa ionik berbentuk padatan putih yang tersusun dari kation natrium Na dan anion hidroksida OH. 2HClaq Zns arrow ZnCl2aq H2g View Answer What volume of H2 gas in liters measured at 21 degrees Celsius and 689 torr can be obtained by reacting 629 grams of zinc metal with 131 mL of.
Aq Ca2aq 2CI-aq CaSO4s 2Naaq 2CI-aq Recall that spectator ions are ions present in solution that do not participate directly in the reaction.

Use Oxidation States To Identify The Oxidizing Agent And The Reducing Agent In The Following Redox Reaction 2na S 2h2o L 2naoh Aq H2 G Reducing Agent Oxidizing Agent Homework Study Com

Solved Consider The Reaction 2na S 2h2o L 2naoh Aq Chegg Com

Which Is The Oxidising Agent In The Following Equation Haso2 Aq Sn 2 Aq H Aq As S Sn 4 Aq H2o I

Solved Express The Equilibrium Constant For The Following Chegg Com

Solved For The Reaction 2na S 2h2o 1 2naoh Aq H2 G Chegg Com
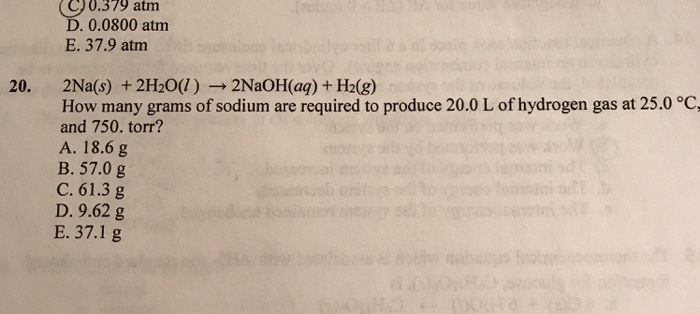
Solved 0 379 Atm 0 0800 Atm E 37 9 Atm 2na S 2h2o L Chegg Com
0 Response to "2na S 2h2o L 2naoh Aq H2 G"
Post a Comment